Multiple Choice
The radial portion of the de Broglie wavefunction for an electron in the ground state of the hydrogen atom is 1s(r) = 1/() 1/2 exp(-r/a0) where a0 is the Bohr radius.The probability of finding the electron is: 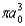
A) 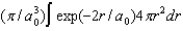
B) 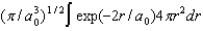
C) 
D) 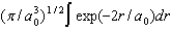
E) 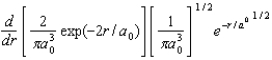
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: A hydrogen atom is in its first
Q13: When using the Pauli Exclusion Principle,
Q14: In a shell of the hydrogen atom
Q15: All quantum states forming a sub-shell have
Q18: The magnitude of the spin angular
Q19: For the following allowed transitions, which photon
Q20: The allowed values of for the n
Q21: In the subshell of the Li2+ ion
Q22: The probability density of a particle at
Q42: Aline says that the magnetic moment of