Multiple Choice
The K, L, M symbols represent values of the quantum number:
A) n
B) 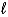
C) 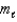
D) ms
E) mj
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Of the following states, 5s, 3p, 4f,
Q19: Forbidden transitions and selection rules suggest that<br>A)
Q21: In the subshell of the Li2+ ion
Q22: The probability density of a particle at
Q23: Characteristic x-rays can be produced by bombarding
Q25: The number of electrons in the n
Q27: The energy needed to remove an electron
Q28: The number of states in the He+
Q35: An electron in a hydrogen atom makes
Q56: In the operation of a laser<br>A) stimulated