Multiple Choice
In the subshell of the Li2+ ion with orbital quantum number 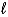 , the allowed values of the magnetic quantum number
, the allowed values of the magnetic quantum number  are:
are:
A) (-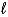 to
to 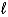 )
)
B) (-( 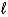 + 1) to (
+ 1) to ( 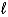 + l)
+ l)
C) (-( 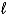 + 2) to (
+ 2) to (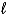 + 2)
+ 2)
D) (-( 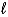 + 3) to (
+ 3) to ( 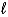 + 3)
+ 3)
E) 0 to n -1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: The radial portion of the de
Q18: The magnitude of the spin angular
Q19: For the following allowed transitions, which photon
Q19: Forbidden transitions and selection rules suggest that<br>A)
Q20: The allowed values of for the n
Q22: The probability density of a particle at
Q23: Characteristic x-rays can be produced by bombarding
Q25: The number of electrons in the n
Q26: The K, L, M symbols represent values
Q42: Aline says that the magnetic moment of