Multiple Choice
If the rms speed of helium atoms is vrms,He at temperature T, what is the rms speed of CO2 at the same temperature?
A) 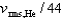
B) 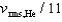
C) 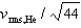
D) 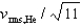
E) 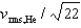
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: During an adiabatic compression,a volume of
Q15: Assume 3.0 moles of a diatomic gas
Q23: Air expands adiabatically (no heat in,no
Q26: The average molecular translational kinetic energy
Q27: The relation PV = nRT holds
Q30: A 50-gram sample of dry ice
Q31: A molecule in a uniform ideal
Q32: Suppose a box contains about 5.0*
Q34: The air in an automobile engine
Q38: One mole of helium gas expands