Multiple Choice
Consider the reaction: 2A + B → C, 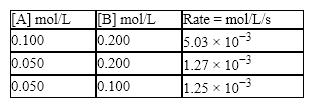 What is the value of the rate constant?
What is the value of the rate constant?
A) 3.20 mol L − 1s
B) 19.8 mol L − 1s
C) 0.530 mol L − 1s
D) 4.60 mol L − 1s
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: The rate constant "k" will vary with
Q31: O<sub>3</sub>, not O<sub>2</sub>, is the thermodynamically favored
Q32: The catalytic converter on an automobile has
Q33: Lowering the activation energy of a reaction
Q34: If the decomposition of reactant A follows
Q35: The synthesis of ammonia from hydrogen and
Q36: Raising the temperature of a reaction elevates
Q37: A termolecular step in a reaction mechanism
Q38: A chemical species that is not consumed
Q39: Sulfur trioxide production follows the reaction:<br>2 SO<sub>2</sub>(