Multiple Choice
Given a Galvanic cell: 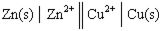 , the right-hand side of this notation represents the:
, the right-hand side of this notation represents the:
A) spontaneous half of the reaction.
B) oxidation 1/2 reaction.
C) anode of the cell.
D) reduction 1/2 reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: The standard state of an electrochemical cell
Q15: Using the provided standard reduction potentials, which
Q16: How many grams of silver are deposited
Q17: A Galvanic cell is an electrochemical cell
Q18: Reduction occurs at the anode of a
Q20: The standard hydrogen electrode (SHE) involves gaseous
Q21: Electrolysis involves using an external current to
Q22: Electrolysis can be used to electroplate metals
Q23: The formation of thin layers of material
Q24: All dry cell batteries are rechargeable.