Multiple Choice
Using the provided standard reduction potentials, which of the following cells is an example of a Galvanic cell?
A) 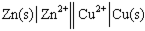
B) 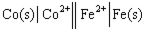
C) 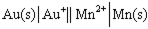
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: What is the E<sup>0</sup> value for the
Q11: Secondary cells are designed to be rechargeable.
Q12: The rusting witnessed in the sheet metal
Q13: What is the oxidation number of Mn
Q14: The standard state of an electrochemical cell
Q16: How many grams of silver are deposited
Q17: A Galvanic cell is an electrochemical cell
Q18: Reduction occurs at the anode of a
Q19: Given a Galvanic cell: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8709/.jpg" alt="Given
Q20: The standard hydrogen electrode (SHE) involves gaseous