Multiple Choice
What is the E value for the Galvanic cell formed from these two half-reactions at these concentrations?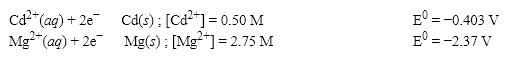
A) 1.90 V
B) 1.95 V
C) 1.99 V
D) 2.20 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: The standard hydrogen electrode (SHE) involves gaseous
Q21: Electrolysis involves using an external current to
Q22: Electrolysis can be used to electroplate metals
Q23: The formation of thin layers of material
Q24: All dry cell batteries are rechargeable.
Q26: Copper has a greater tendency to corrode
Q27: An oxidation occurs at the _ of
Q28: What is the E value for the
Q29: Which mechanism is most energy efficient?<br>A) internal
Q30: The Nernst equation describes the pH required