Multiple Choice
What is the E value for the Galvanic cell formed from these two half-reactions at these concentrations?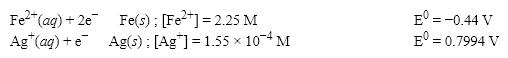
A) 1.00 V
B) 1.12 V
C) 1.24 V
D) 0.36 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: The formation of thin layers of material
Q24: All dry cell batteries are rechargeable.
Q25: What is the E value for the
Q26: Copper has a greater tendency to corrode
Q27: An oxidation occurs at the _ of
Q29: Which mechanism is most energy efficient?<br>A) internal
Q30: The Nernst equation describes the pH required
Q31: The species undergoing reduction is referred to
Q32: Single use, non-rechargeable batteries are referred to
Q33: How long will it take to collect