Multiple Choice
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 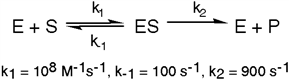
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the equilibrium constant for the enzyme-catalyzed reaction?
A) 0.9
B) 1.1
C) 2.5
D) Cannot be determined from the information provided.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Which of the following is true?<br>A) The
Q14: According to the steady-state assumption<br>A) the product
Q15: Enzymatic activity has an optimum temperature because<br>A)
Q16: Exhibit 6A This is a reaction going
Q17: Exhibit 6A This is a reaction going
Q19: Exhibit 6A This is a reaction going
Q20: The amount of energy released during a
Q21: Irreversible inhibitors of enzymatic reactions<br>A) bind to
Q22: The Michaelis-Menten approach to describing the kinetics
Q23: Inhibitors can have the following effects on