Multiple Choice
Consider the following geometric shapes. Which one is a tetrahedral?
A) 
B) 
C) 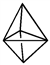
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q84: Which of the following pairs of elements
Q85: Which of the following covalent molecules contains
Q86: The correct name for K<sub>2</sub>SO<sub>4</sub> is _
Q87: Knowing that indium is a member of
Q88: Eating foods rich in _ has been
Q89: Which of the following correctly illustrates hydrogen
Q90: CCl<sub>4</sub> has a tetrahedral structure.
Q91: The rigid three-dimensional arrangement assumed by the
Q92: Explain the rules that should be
Q93: Which type of interparticle forces leads to