Multiple Choice
Identify all Brønsted base(s) in the reaction. N3- (aq) + H2O (l)  HN3 (aq) + OH- (aq)
HN3 (aq) + OH- (aq)
A) H2O
B) 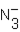
C) OH-
D) 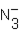 and OH-
and OH-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q69: A 10<sup>-8</sup> M solution of HCl is
Q70: Which of the following is a property
Q71: The dissociation reaction for the weak acid,
Q72: Which of the following would you expect
Q73: A reaction in which an acid and
Q75: Most acids are weak acids.
Q76: When your liver detoxifies ethyl alcohol, the
Q77: The cation (positive ion)in a salt comes
Q78: The pH of household ammonia is expected
Q79: Which of the following mixtures would represent