Multiple Choice
The dissociation reaction for the weak acid, H2PO3- would be which of the following?
A) 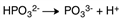
B) 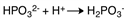
C) 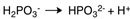
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q66: Identify the Brønsted acid(s)in the reaction. HIO<sub>3</sub>(aq)+
Q67: A patient comes to you suffering from
Q68: Seageroic acid has a p K <sub>a</sub>
Q69: A 10<sup>-8</sup> M solution of HCl is
Q70: Which of the following is a property
Q72: Which of the following would you expect
Q73: A reaction in which an acid and
Q74: Identify all Brønsted base(s)in the reaction. N<sub>3</sub><sup>-</sup>
Q75: Most acids are weak acids.
Q76: When your liver detoxifies ethyl alcohol, the