Multiple Choice
The possible values of the magnetic quantum number, 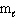 , of a 3d electron are:
, of a 3d electron are:
A) 1, 2, 3
B) 0, 1, 2
C) - 2, - 1, 0, 1, 2
D) - 1, 0, 1
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Which orbital diagram for the ground state
Q28: How many electrons can be placed in
Q29: What is the maximum number of electrons
Q30: A subshell is described by assigning values
Q31: What is the correct electronic configuration for
Q33: Which subshell can hold up to a
Q34: Which equation below properly relates energy (E)
Q35: The element with a ground state electron
Q36: The distance between two adjacent peaks of
Q37: The possible values of the magnetic quantum