Multiple Choice
The possible values of the magnetic quantum number, 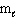 , of a 2p electron are:
, of a 2p electron are:
A) - 1, 0, 1
B) - 2, - 1, 0, 1, 2
C) 0, 1, 2
D) 1, 2, 3
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: The possible values of the magnetic quantum
Q33: Which subshell can hold up to a
Q34: Which equation below properly relates energy (E)
Q35: The element with a ground state electron
Q36: The distance between two adjacent peaks of
Q38: Calculate the frequency of light whose wavelength
Q39: The energy of a photon of electromagnetic
Q40: Which atomic subshell can hold up to
Q41: Scientists of the 19th Century found that
Q42: Exhibit 7-1 Consider the shapes listed below