Multiple Choice
How many possible orientations are allowed when 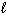 = 1?
= 1?
(p-type sublevel; How many possible 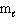 values are possible?)
values are possible?)
A) 1
B) 2
C) 3
D) 5
E) 7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q61: Only one set of quantum numbers listed
Q62: How many electrons can be placed in
Q63: What is the wavelength of light with
Q64: The number of wave crests that pass
Q65: The energy of the electron in the
Q67: The quantum number <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg" alt="The quantum
Q68: According to the Bohr model for a
Q69: Calculate the wavelength of light emitted when
Q70: Given the three statements below, which answer
Q71: Three sets of quantum numbers are listed