Multiple Choice
Three sets of quantum numbers are listed below. Pick the best answer.
I. n = 3, 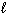 = 3,
= 3, 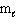 = 2
= 2
II. n = 4, 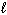 = 2,
= 2, 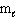 = 0
= 0
III. n = 1, 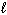 = 0,
= 0, 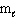 = 0
= 0
A) I and II are allowed sets, III is not
B) only III is an allowed set
C) II and III are allowed sets, I is not
D) all three sets are allowed
E) only II is an allowed set
Correct Answer:

Verified
Correct Answer:
Verified
Q66: How many possible orientations are allowed when
Q67: The quantum number <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg" alt="The quantum
Q68: According to the Bohr model for a
Q69: Calculate the wavelength of light emitted when
Q70: Given the three statements below, which answer
Q72: Given the three statements below, pick the
Q73: Which orbital diagram for the ground state
Q74: Which set(s) of three quantum numbers is(are)
Q75: Exhibit 7-1 Consider the shapes listed below
Q76: Which full set of four quantum numbers