Multiple Choice
Exhibit 7-1 Consider the shapes listed below to answer the following question(s) :
I. 
II. 
III. 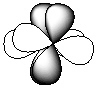
IV. 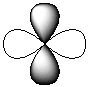
V. 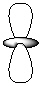
-Refer to Exhibit 7-1. Which of the shapes would represent one of seven possible 4f atomic orbitals?
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Q37: The possible values of the magnetic quantum
Q38: Calculate the frequency of light whose wavelength
Q39: The energy of a photon of electromagnetic
Q40: Which atomic subshell can hold up to
Q41: Scientists of the 19th Century found that
Q43: What is the correct electronic configuration for
Q44: What is the energy of one photon
Q45: Which neutral element has an electronic configuration
Q46: What quantum number(s) provide(s) information with respect
Q47: What quantum number(s) is(are) required to completely