Multiple Choice
Which full set of four quantum numbers would be expected for the highest energy electron for a potassium atom?
A) n = 4, 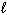 = 0,
= 0, 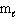 = 0, m s = +1/2
= 0, m s = +1/2
B) n = 4, 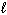 = 1,
= 1, 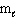 = 1, m s = +1/2
= 1, m s = +1/2
C) n = 4, 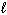 = 2,
= 2,  = 2, m s = +1/2
= 2, m s = +1/2
D) n = 3, 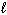 = 1,
= 1,  = 1, m s = +1/2
= 1, m s = +1/2
E) n = 3, 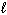 = 2,
= 2, 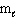 = 2, m s = +1/2
= 2, m s = +1/2
Correct Answer:

Verified
Correct Answer:
Verified
Q71: Three sets of quantum numbers are listed
Q72: Given the three statements below, pick the
Q73: Which orbital diagram for the ground state
Q74: Which set(s) of three quantum numbers is(are)
Q75: Exhibit 7-1 Consider the shapes listed below
Q77: From the restrictions on the three quantum
Q78: What is the ground state electron configuration
Q79: What is the correct electronic configuration for
Q80: What is the order for filling the
Q81: What is the wavelength of an electron