Multiple Choice
The valence shell orbital configuration of the Si atom in its ground state is:
A) 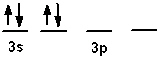
B) 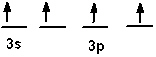
C) 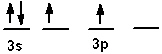
D) 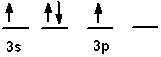
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: The answer which correctly arranges the species
Q3: What ion has the ground state electron
Q4: Three statements are listed below. Pick the
Q5: How many valence electrons are present in
Q6: Which element has the valence electrons 3s<sup>2</sup>3p<sup>2</sup>?<br>A)
Q8: How many unpaired electrons does the ground
Q9: What is the order for increasing atomic
Q10: The ground state electron configuration 1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>2</sup> would
Q11: Which species is isoelectronic with As?<br>A) Ga<sup>2+</sup><br>B)
Q12: The highest energy electron for Ga (Z