Multiple Choice
The highest energy electron for Ga (Z = 31) could be designated by which of the following four quantum numbers (only one correct possibility is shown, others are possible) ?
A) n = 3, 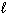 = 1,
= 1, 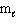 = 1, m s = 1/2
= 1, m s = 1/2
B) n = 4, 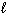 = 1,
= 1, 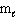 = - 1, m s = 1/2
= - 1, m s = 1/2
C) n = 3, 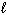 = 0,
= 0, 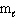 = 0, m s = 1/2
= 0, m s = 1/2
D) n = 4, 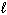 = 2,
= 2, 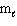 = 1, m s = 1/2
= 1, m s = 1/2
E) n = 5, 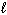 = 2,
= 2, 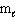 = 1, m s = 1/2
= 1, m s = 1/2
Correct Answer:

Verified
Correct Answer:
Verified
Q7: The valence shell orbital configuration of the
Q8: How many unpaired electrons does the ground
Q9: What is the order for increasing atomic
Q10: The ground state electron configuration 1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>2</sup> would
Q11: Which species is isoelectronic with As?<br>A) Ga<sup>2+</sup><br>B)
Q13: Which of the following is an acceptable
Q14: The ground state electron configuration of Co
Q15: Arrange the following species in order of
Q16: Arrange the following elements in order of
Q17: Which answer below correctly lists the atoms