Multiple Choice
What intermolecular force(s) of attraction is(are) present between two molecules of ethane shown below?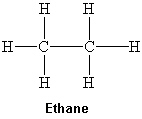
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q67: Aluminum metal crystallizes in a face centered
Q68: A metallic element crystallizes in a face-centered
Q69: Exhibit 11-2 The phase diagram below is
Q70: Based upon intermolecular forces of attraction, which
Q71: Exhibit 11-1 The heating curve below is
Q73: Which of the following phase transitions are
Q74: In a cubic closest packing array of
Q75: The term that best explains why water
Q76: Which of the following would have a
Q77: Which of the three states of matter