Multiple Choice
Aluminum metal crystallizes in a face centered cubic cell . One face of this unit cell is shown in the diagram that follows. The length along one edge equals 4.04 . 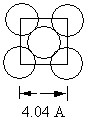 What is the atomic radius of an aluminum atom?
What is the atomic radius of an aluminum atom?
A) 1.43 Å
B) 2.02 Å
C) 2.86 Å
D) 5.72 Å
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Q62: A metal that crystallizes in a face-centered
Q63: Exhibit 11-3 Consider the General Phase Diagram
Q64: Which molecule listed below would have its
Q65: A diamond crystal is classified as:<br>A) ionic<br>B)
Q66: A crystal diffracts x-rays (£ = 154
Q68: A metallic element crystallizes in a face-centered
Q69: Exhibit 11-2 The phase diagram below is
Q70: Based upon intermolecular forces of attraction, which
Q71: Exhibit 11-1 The heating curve below is
Q72: What intermolecular force(s) of attraction is(are) present