Multiple Choice
What volume of 0.200 M H₂ SO₄is required to neutralize 50.0 mL of 0.100 M NaOH?
Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O ( 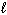 )
)
A) 50.0 mL
B) 25.0 mL
C) 100 mL
D) 12.5 mL
E) 6.25 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: A buffer was prepared such that the
Q36: Calculate the pH of a solution formed
Q37: Which of the following mixtures would not
Q38: A sample of 0.100 mole of acetic
Q39: Consider the titration of a weak acid
Q41: What is the pH of an aqueous
Q42: Consider the following equilibrium reaction:<br>NH₃+ H₂ O
Q43: Calculate the pH of a solution that
Q44: A 25.0 mL volume of 0.100 M
Q45: A 25.0 mL volume of a 0.200