Multiple Choice
Consider the following equilibrium reaction:
NH₃+ H₂ O 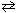 NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
(Use Le Chatelier s principle.)
A) The equilibrium will shift left favoring the reactant side and the pH will drop.
B) The equilibrium will shift right favoring the product side and the pH will drop.
C) There will be no shift in the equilibrium reaction and the pH will remain constant.
D) The equilibrium will shift left favoring the reactant side and the pH will rise.
E) The equilibrium will shift right favoring the product side and the pH will rise.
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Which of the following mixtures would not
Q38: A sample of 0.100 mole of acetic
Q39: Consider the titration of a weak acid
Q40: What volume of 0.200 M H₂ SO₄is
Q41: What is the pH of an aqueous
Q43: Calculate the pH of a solution that
Q44: A 25.0 mL volume of 0.100 M
Q45: A 25.0 mL volume of a 0.200
Q46: What is the pH of the following
Q47: If it takes 32.0 mL of 0.100