Multiple Choice
Consider the ionization of acetic acid as follows:CH ₃COOH + H₂ O 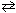 H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:
H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:
CH₃COONa) , to this equilibrium system?
A) The equilibrium shifts to the left and the pH rises.
B) The equilibrium shifts to the right and the pH rises.
C) The equilibrium shifts to the left and the pH lowers.
D) The equilibrium shifts to the right and the pH lowers.
E) Nothing happens. The pH remains constant and the equilibrium does not shift in either direction.
Correct Answer:

Verified
Correct Answer:
Verified
Q87: What is the concentration of ammonium ion,
Q88: The sharpest inflection point is observed in
Q89: In the titration of 0.100 M HCl
Q90: The titration of a weak acid, HA
Q91: What is the pH of solution formed
Q93: Exhibit 16-3 Consider the Titration curve below
Q94: A solution is made by dissolving 0.100
Q95: Calculate the pH at the equivalence point
Q96: Carbonic acid is a diprotic acid with
Q97: A sample of hydrochloric acid (approximately 0.10