Multiple Choice
Exhibit 16-3 Consider the Titration curve below for the titration of a weak acid ,CH ₃COOH , with a strong base , NaOH, to answer the following question(s) . CH ₃COOH + NaOH CH ₃COONa + H₂ O 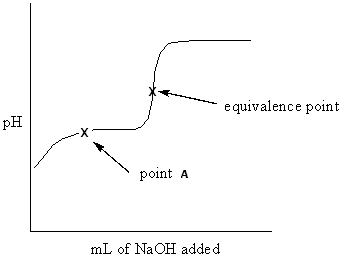
Refer to Exhibit 16-3. What approach listed below would be used to determine the pH at point A of the solution?
A) This is a problem of solving for the pH of an aqueous weak acid solution.
B) This is a problem of solving for the pH of an aqueous weak base solution.
C) This is a problem of solving for the pH of an aqueous buffer solution.
D) This is a problem of solving for the pH of an aqueous strong base solution.
E) This is a problem of solving for the pH of an aqueous salt solution.
Correct Answer:

Verified
Correct Answer:
Verified
Q88: The sharpest inflection point is observed in
Q89: In the titration of 0.100 M HCl
Q90: The titration of a weak acid, HA
Q91: What is the pH of solution formed
Q92: Consider the ionization of acetic acid as
Q94: A solution is made by dissolving 0.100
Q95: Calculate the pH at the equivalence point
Q96: Carbonic acid is a diprotic acid with
Q97: A sample of hydrochloric acid (approximately 0.10
Q98: The titration of a weak base (