Multiple Choice
Boyle's law states that for low pressures, the pressure of an ideal gas kept at a constant temperature varies inversely with the volume of the gas. State Boyle's law as an equation using the symbols P , K , and V .
A) 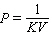
B) 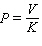
C) 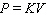
D) 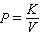
E) 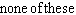
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q139: Find the equation of the line parallel
Q140: Let f ( x ) = 10
Q141: The following graph shows the relationship between
Q142: In an aerobics class, the instructor indicates
Q143: Give the slope and y -intercept for
Q145: Graph the following relation. Use the graph
Q146: Give the equation of the line perpendicular
Q147: The relative aperture or f -stop (
Q148: Find the equation of the line with
Q149: In an aerobics class, the instructor indicates