Multiple Choice
Saline solutions (NaCl in water) used to deliver intravenous drugs are 0.89%(w/v) . What mass of NaCl would be needed to prepare 450.0 mL of such a solution?
A) 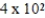 g
g
B) 0.89 g
C) 4.0 g
D) 5.1 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Consider the solutions shown in the containers
Q11: Consider the two containers separated by a
Q18: The vitamins A (retinol)and C (ascorbic acid)are
Q26: What is the mass of salt in
Q45: For the following questions,fill in the blank
Q50: Consider the two containers separated by a
Q51: For a 2.00 M solution, the conversion
Q55: Solution contains 55 mg of magnesium in
Q59: Calculate the number of moles of ZnCl<sub>2</sub>,
Q60: For the following questions,fill in the blank