Multiple Choice
Calculate the number of moles of ZnCl2, in 180.0 mL of 0.330 M solution.
A) 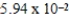 mol
mol
B) 1.83 mol
C) 0.545 mol
D) 59.4 mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Consider the two containers separated by a
Q18: The vitamins A (retinol)and C (ascorbic acid)are
Q26: What is the mass of salt in
Q42: Consider the following structure. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt="Consider
Q43: Consider two solutions,A and B,separated by a
Q45: For the following questions,fill in the blank
Q50: Consider the two containers separated by a
Q56: Saline solutions (NaCl in water) used to
Q60: For the following questions,fill in the blank
Q70: Lactated Ringer's solution 109 mEq/L of Cl<sup>-</sup>.