Multiple Choice
If we know the amount of light that is absorbed in a solution, we can calculate the concentration of the substance dissolved in water using a spectrophotometer. For a certain substance the concentration (in moles/liter) is given by the formula  , where
, where 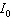 is the intensity of the incident light and
is the intensity of the incident light and 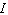 is the intensity of light that emerges. What is the concentration of the substance if the intensity
is the intensity of light that emerges. What is the concentration of the substance if the intensity 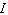 is 45% of
is 45% of 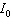 ?
?
A) 952 mol/L
B) -3,667 mol/L
C) 2,928 mol/L
D) 2,192 mol/L
E) 1,272 mol/L
Correct Answer:

Verified
Correct Answer:
Verified
Q64: Solve the logarithmic equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8728/.jpg" alt="Solve
Q65: Solve the logarithmic equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8728/.jpg" alt="Solve
Q66: Find <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8728/.jpg" alt="Find .
Q67: Which one of the following is the
Q68: Using the graph below, determine which one
Q70: Which one of the following is the
Q71: Use the Change of Base Formula to
Q72: Two functions and are given by the
Q73: Which one of the following is the
Q207: If $18000 is invested at an interest