Multiple Choice
The oxylate ion is a 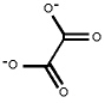
A) monodentate ligand.
B) bidentate ligand.
C) tridentate ligand.
D) tetradentate ligand.
E) will not act as a metal ligand.
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Generally speaking which is the least stable
Q9: Name the following compound, Pt(H<sub>2</sub>NCH<sub>2</sub>CH<sub>2</sub>NH<sub>2</sub>)Cl<sub>4</sub>]Cl<sub>2</sub><br>A) hexachloro(ethylenediamine)platinum (IV)<br>B)
Q10: What is the oxidation state of Cr
Q11: Name the following compound, Na<sub>2</sub>[NiCl<sub>4</sub>].<br>A) disodium tetrachloronickel
Q12: Using the Lewis classification of acids and
Q14: Would it be better to use Cr<sup>3+</sup>
Q15: How many isomers are there of the
Q16: In the VB picture what is the
Q17: What can you say about the following
Q18: How many isomers are there of trisoxaltochromate