Multiple Choice
What can you say about the following two compounds? 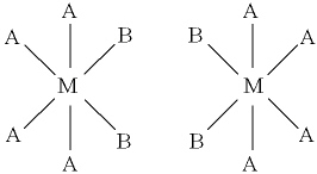
A) they are isomers
B) they are enantiomers
C) they are isomers and enantiomers
D) they are the identical compounds
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Using the Lewis classification of acids and
Q13: The oxylate ion is a <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10703/.jpg"
Q14: Would it be better to use Cr<sup>3+</sup>
Q15: How many isomers are there of the
Q16: In the VB picture what is the
Q18: How many isomers are there of trisoxaltochromate
Q19: A Co<sup>2+</sup> ion in a weak octahedral
Q20: What is the crystal field stabilization energy
Q21: Why are Zn<sup>2+</sup> ions colorless in water?<br>A)
Q22: The octahedral complex [Co(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup> has an absorption