Essay
A student allowed an alcohol, 4-penten-1-ol, to react with Br2, expecting to observe a simple addition of Br2 to the double bond. Instead, she isolated a compound X that proved to have the formula C5H9OBr. The reaction mixture from which X was isolated was very acidic.
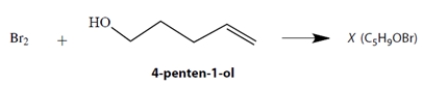 Using mechanistic reasoning, including the curved-arrow notation, propose a structure for compound X. Show your logic at every step of the way! Your answer should explain why the reaction mixture became acidic.
Using mechanistic reasoning, including the curved-arrow notation, propose a structure for compound X. Show your logic at every step of the way! Your answer should explain why the reaction mixture became acidic.
Correct Answer:

Verified
When alkenes react with bromine, typical...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Devise a synthetic route for this conversion.
Q2: Compare the results of hydroboration-oxidation and Hg<sup>2+</sup>-catalyzed
Q3: Draw the structures of three enols that
Q5: Complete the reaction by providing the structure
Q6: Give the structure of the enol intermediate
Q7: Consider the reaction, in which X is
Q8: Devise a synthetic route for this conversion.<br>
Q9: Identify the reagents needed to transform isopentene
Q10: Complete the reaction by providing the structure
Q11: Complete the reaction by providing the structure