Essay
Consider the reaction, in which X is a stable, mercury-containing compound (not a reactive intermediate). Compound Y does not react with either Br2, H2/catalyst, or ozone.
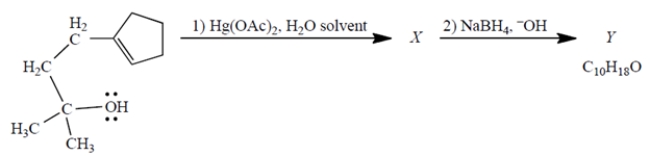 a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.
a. What do you deduce about the structure of Y solely from its formula and the reactivity (or "unreactivity") data give? Explain briefly.
b. Use mechanistic reasoning to deduce the structure of X. Use the curved-arrow notation; show all relevant unshared pairs and charges. Then, using what you know about step (2)-no mechanisms!-deduce the structure of the product Y.
Correct Answer:

Verified
a. The reactivity data shows that the me...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q2: Compare the results of hydroboration-oxidation and Hg<sup>2+</sup>-catalyzed
Q3: Draw the structures of three enols that
Q4: A student allowed an alcohol, 4-penten-1-ol, to
Q5: Complete the reaction by providing the structure
Q6: Give the structure of the enol intermediate
Q8: Devise a synthetic route for this conversion.<br>
Q9: Identify the reagents needed to transform isopentene
Q10: Complete the reaction by providing the structure
Q11: Complete the reaction by providing the structure
Q12: Complete the reaction by providing the missing