Essay
Ascorbic acid (Vitamin C) is a diprotic acid with two pKa values; pKa1 = 4.2 and pKa2 = 11.6. The two acidic groups are the two -OH groups, indicated by arrows in the structure. If we let AH2 represent ascorbic acid, its successive ionized species can be abbreviated by −AH and A2−.
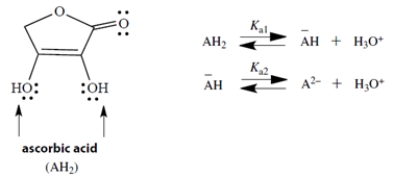 Draw the structure of −AH and its two resonance structures. Draw the curved arrow notation to convert each structure to the next. (Hint: In one structure, charge is delocalized into the C=O group.)
Draw the structure of −AH and its two resonance structures. Draw the curved arrow notation to convert each structure to the next. (Hint: In one structure, charge is delocalized into the C=O group.)
Correct Answer:

Verified
Your first task is to decide which OH gr...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q6: Consider the acid-base equilibrium:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider
Q7: Show the curved-arrow notation and the product
Q8: Select the strongest acid.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Select the
Q9: Select the equilibrium that most favors
Q10: Choose the number that is closest to
Q12: The dissociation reaction for an acid has
Q13: Consider the Brønsted acid-base reaction:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg"
Q14: In this acid-base equilibrium, the pK<sub>a</sub>
Q15: Select the correct statement about this
Q16: Draw curved arrows and give the products