Essay
Show the curved-arrow notation and the product (including all unshared valence electrons and formal charges, if any) for the Lewis acid-base association reaction of the two species. (Aluminum is in group 3A of the periodic table, beneath boron.) Then identify and label the nucleophile in the reaction.
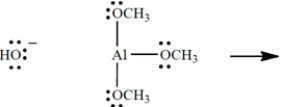
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The free energy of formation (ΔG<sub>f</sub>°) is
Q3: Aspirin has the structure on the left
Q4: Give the products of the electron-pair displacement
Q5: Using curved arrows, show how each resonance
Q6: Consider the acid-base equilibrium:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider
Q8: Select the strongest acid.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Select the
Q9: Select the equilibrium that most favors
Q10: Choose the number that is closest to
Q11: Ascorbic acid (Vitamin C) is a diprotic
Q12: The dissociation reaction for an acid has