Essay
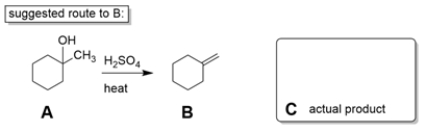
Two students working in a laboratory are tasked to synthesize compound B from compound A. The first student thinks that a dehydration reaction, treating A with strong acid and heat, will do the trick. The second student disagrees and says the reaction will not work as intended.
a. Explain why the second student is right and predict the more likely product (C) of the reaction.
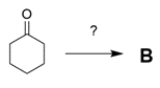
b. If you could start with cyclohexanone, how would you prepare B? You may use any other reagents you want. Show all steps and intermediates, but reaction mechanisms are not necessary.
Correct Answer:

Verified
a. The alcohol dehydration is an E1 reac...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Identify the missing reagent for the reaction.<br>
Q2: The conversion of alkyl azides into primary
Q3: Provide a detailed, arrow-pushing mechanism for the
Q4: Predict the major organic product in the
Q6: Identify the missing reagent for the reaction.<br>
Q7: Deduce the structure of the missing starting
Q8: For each pair of reactions, circle "faster"
Q9: Outline a synthesis to achieve this transformation.<br>
Q10: Predict the major organic product of the
Q11: Treatment of a mixture of cyclopentanone (A)