Essay
The conversion of alkyl azides into primary amines through a procedure called the Staudinger reaction is shown.
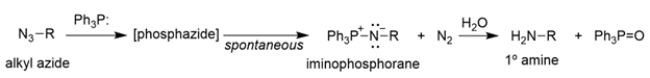 The mechanism of the first part of the reaction is conceptually similar to the Wittig reaction that we've talked about, where an ylide reacts with a carbonyl to yield an oxaphosphetane, which then rearranges to an alkene and triphenylphosphine oxide.
The mechanism of the first part of the reaction is conceptually similar to the Wittig reaction that we've talked about, where an ylide reacts with a carbonyl to yield an oxaphosphetane, which then rearranges to an alkene and triphenylphosphine oxide.
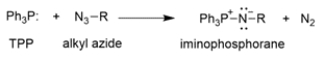
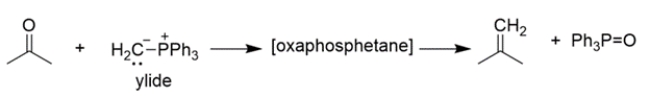 Provide an arrow pushing mechanism for the first part of the Staudinger reaction, the reaction of triphenylphosphine (TPP) and an alkyl azide to produce an iminophosphorane and nitrogen gas.
Provide an arrow pushing mechanism for the first part of the Staudinger reaction, the reaction of triphenylphosphine (TPP) and an alkyl azide to produce an iminophosphorane and nitrogen gas.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Identify the missing reagent for the reaction.<br>
Q3: Provide a detailed, arrow-pushing mechanism for the
Q4: Predict the major organic product in the
Q5: Two students working in a laboratory are
Q6: Identify the missing reagent for the reaction.<br>
Q7: Deduce the structure of the missing starting
Q8: For each pair of reactions, circle "faster"
Q9: Outline a synthesis to achieve this transformation.<br>
Q10: Predict the major organic product of the
Q11: Treatment of a mixture of cyclopentanone (A)