Essay
The rate of formation of the oxime functional group from acetone and hydroxylamine in water is pH dependent. The ideal pH for this reaction is about 4.8.
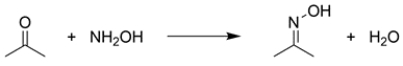 a. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 1.
a. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 1.
b. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 8.
Correct Answer:

Verified
a. At pH 1, the electrophile is activate...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Provide detailed, arrow-pushing mechanisms for the two-step
Q16: Outline a synthesis for the transformation. Identify
Q17: In the reaction of H<sub>2</sub>O at pH
Q18: In each set of reactions, circle the
Q19: Draw the structure of the major organic
Q20: Predict the major organic product for the
Q21: Shown are two acetals. In the corresponding
Q22: Predict the major organic product of the
Q23: Bases can catalyze nucleophilic addition reactions of
Q24: <font face="symbol"></font>-Methoxyglucose contains which functional group?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg"