Essay
1,3-Cyclopentadiene is unusually acidic, with a pKa of ~16. On the other hand, 1,3,5-cyclohepatriene is much less acidic, with a pKa of ~36. Explain why the compounds have such differing acidities.
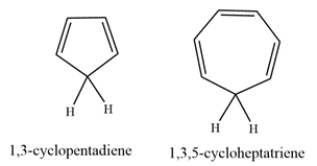
Correct Answer:

Verified
Deprotonation of one of the sp3 hydrogens...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q12: Draw the major organic product of this
Q13: Which compound or ion has a UV/visible
Q14: Which of these compounds is aromatic?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg"
Q15: Determine whether each compound is aromatic, antiaromatic,
Q16: Draw a Frost circle and determine the
Q18: Select all structures that contain conjugated pi
Q19: Pyridine and pyrrole are both aromatic nitrogen-containing
Q20: The Diels-Alder reaction can give two constitutional
Q21: Which compound or ion has five <font
Q22: Cyclooctatetraene (COT) has eight pi electrons but