Multiple Choice
Which compound or ion has five molecular orbitals?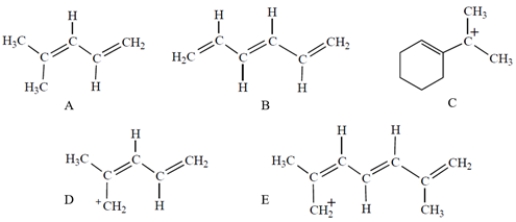
A) compound A
B) compound B
C) compound C
D) compound D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: Determine whether each compound is aromatic, antiaromatic,
Q16: Draw a Frost circle and determine the
Q17: 1,3-Cyclopentadiene is unusually acidic, with a pK<sub>a</sub>
Q18: Select all structures that contain conjugated pi
Q19: Pyridine and pyrrole are both aromatic nitrogen-containing
Q20: The Diels-Alder reaction can give two constitutional
Q22: Cyclooctatetraene (COT) has eight pi electrons but
Q23: Which compound should have the greatest <font
Q24: 1,3-Butadiene, the simplest conjugated diene, has a
Q25: Which compound has the smaller (less positive