Multiple Choice
A compound X has a formula C7H16O4 and the following NMR spectrum, given as chemical shift (integral, splitting, coupling constant) .
1.93 (2H, t, J = 6 Hz) ; 3.35 (12H, s) ; 4.49 (2H, t, J = 6 Hz)
Which structure is consistent with this NMR spectrum?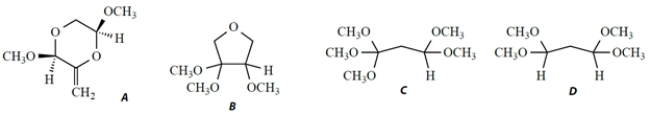
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E
F) compound F
G) compound G
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Deduce the structure of an unknown compound
Q17: The NMR spectrum of the missing compound
Q18: Which letter corresponds to the set of
Q19: Consider the bolded protons in 1-propanol.<br>
Q20: Deduce the structure of a compound C<sub>6</sub>H<sub>14</sub>O
Q22: Explain how you could use proton decoupled
Q23: Deduce the structure of a compound with
Q24: The spectra were recorded for a compound
Q25: The resonance for the methyl group in
Q26: 7. Consider the NMR spectrum of the