Essay
The spectra were recorded for a compound with a molecular formula C5H10O2. Deduce the structure of this compound and explain how you arrive at the correct structure of the compound. (Hint: There are diastereotopic protons in this compound.) NOTE: In the NMR, after a D2O shake, the triplet at 3.27 disappears, and the doublet at 3.7 becomes a singlet.
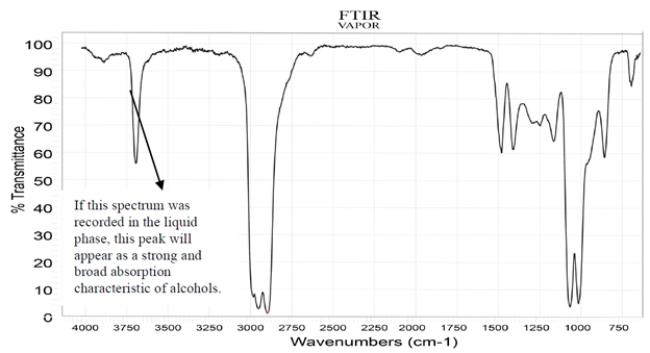
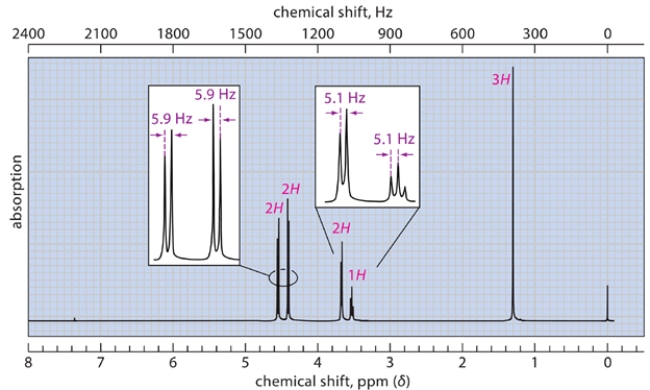
Correct Answer:

Verified
First calculate the degree of unsaturati...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q17: The NMR spectrum of the missing compound
Q18: Which letter corresponds to the set of
Q19: Consider the bolded protons in 1-propanol.<br>
Q20: Deduce the structure of a compound C<sub>6</sub>H<sub>14</sub>O
Q21: A compound X has a formula C<sub>7</sub>H<sub>16</sub>O<sub>4</sub>
Q22: Explain how you could use proton decoupled
Q23: Deduce the structure of a compound with
Q25: The resonance for the methyl group in
Q26: 7. Consider the NMR spectrum of the
Q27: 11. Consider the ether:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="11.