Essay
These two compounds have similar molecular weights. Explain how to use mass spectrometry to differentiate between the two.
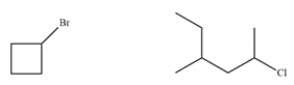
Correct Answer:

Verified
Bromine consists of equal amounts of 79Br ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Bromine consists of equal amounts of 79Br ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q12: Which molecular vibration does not have an
Q13: Outline a synthesis for the transformation. Describe
Q14: Identify the compound that corresponds to the
Q15: The electron ionization mass spectrum of 1-hexanol
Q16: The IR absorption that occurs at the
Q18: The electron ionization of an unknown neutral
Q19: A graduate student is cleaning out the
Q20: Typical IR absorptions occur in the 650-3700
Q21: When the epoxy thiol is treated with
Q22: The base peak of 2,2-dimethylpentane is at