Multiple Choice
The electron ionization of an unknown neutral compound gives this mass spectrum. Which combination of elements most likely comprises the molecule?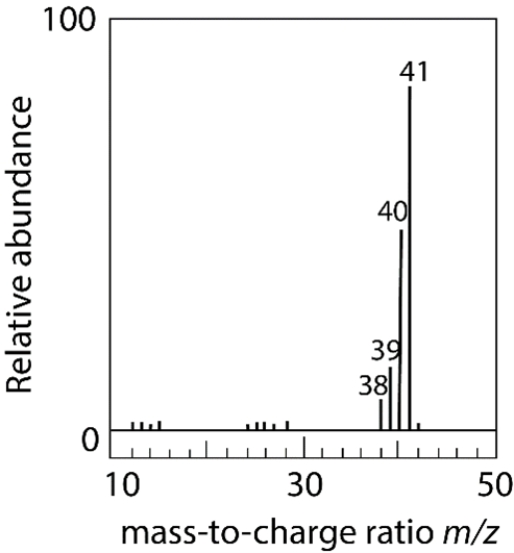
A) two carbons, three hydrogens, and one nitrogen
B) three carbons and five hydrogens
C) one carbon, five hydrogens, and two nitrogen atoms
D) two carbons, four hydrogens, and one nitrogen
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Outline a synthesis for the transformation. Describe
Q14: Identify the compound that corresponds to the
Q15: The electron ionization mass spectrum of 1-hexanol
Q16: The IR absorption that occurs at the
Q17: These two compounds have similar molecular weights.
Q19: A graduate student is cleaning out the
Q20: Typical IR absorptions occur in the 650-3700
Q21: When the epoxy thiol is treated with
Q22: The base peak of 2,2-dimethylpentane is at
Q23: Menthol is obtained from oils of peppermint