Essay
Compound A (C5H11BrO), when dissolved in ethanol containing one equivalent of NaOH, forms a product B. Identify compound A and give a two-step curved-arrow mechanism for its conversion into B. In each step of the curved-arrow mechanism identify the Brønsted acids (BA), Brønsted bases (BB), nucleophiles (N), electrophiles (E), and leaving groups (LG).
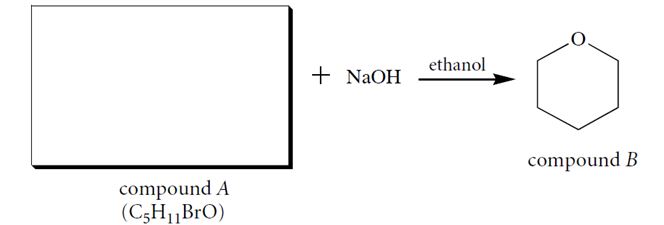
Correct Answer:

Verified
The starting material has no unsaturatio...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Provide the major organic product for the
Q2: Complete the reaction:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Complete the
Q3: Propose a multistep synthesis for the epoxide
Q4: Complete the reaction by giving the major
Q5: Complete the reaction:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Complete the
Q7: Complete the reaction by giving the major
Q8: Using curved-arrow notation, propose a mechanism for
Q9: The reaction fails to generate the indicated
Q10: Outline a synthesis to give the transformation:<br>
Q11: Give the structure of the nucleophile that