Essay
Using curved-arrow notation, propose a mechanism for the transformation that takes into account the stereochemistry of the product. (Hint: Note that two inversions of stereochemistry = one retention.)
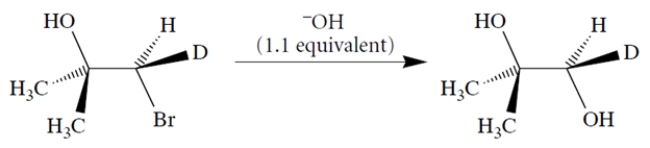
Correct Answer:

Verified
The hint should have called your attenti...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: Propose a multistep synthesis for the epoxide
Q4: Complete the reaction by giving the major
Q5: Complete the reaction:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Complete the
Q6: Compound A (C<sub>5</sub>H<sub>11</sub>BrO), when dissolved in ethanol
Q7: Complete the reaction by giving the major
Q9: The reaction fails to generate the indicated
Q10: Outline a synthesis to give the transformation:<br>
Q11: Give the structure of the nucleophile that
Q12: cis-3-Hexene is treated with OsO<sub>4</sub> (osmium tetroxide),
Q13: Select the one true statement about biological