Solved
Treatment of (+)-(R)-3-Methyl-1-Pentene with HBr and Peroxides Yields (-)-1-Bromo-3-Methyl Pentane
Short Answer
Treatment of (+)-(R)-3-methyl-1-pentene with HBr and peroxides yields (-)-1-bromo-3-methyl pentane. Determine the stereochemical configuration of the product.
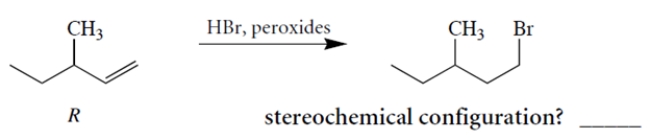
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Give the product A that results when
Q9: 1-Methyl-1-vinyl cyclopentane undergoes addition of HBr under
Q10: A free-radical addition of a certain
Q11: Complete the reaction by giving the major
Q12: Provide the structures of the missing compounds.<br>
Q14: Complete the reaction by giving the missing
Q15: Provide the major organic product for the
Q16: Complete the reactions by giving the structure
Q17: Outline a multi-step synthesis of 4-octanone from
Q18: Consider this addition polymer, called poly(methyl methacrylate).