Essay
Give the product A that results when HBr undergoes peroxide-mediated addition to 1-methylcyclopentene. Also give the structure of the free-radical intermediate B (other than Br.) in the propagation steps of this reaction.
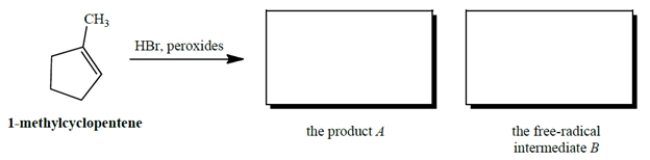
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Identify the stereochemistry of the polymers.<br> <img
Q4: Outline a synthesis of this product.<br> <img
Q5: Free-radical polymerization of ethylene proceeds with the
Q6: Devise a synthesis to achieve the transformation.<br>
Q7: Give the structure of the organic free-radical
Q9: 1-Methyl-1-vinyl cyclopentane undergoes addition of HBr under
Q10: A free-radical addition of a certain
Q11: Complete the reaction by giving the major
Q12: Provide the structures of the missing compounds.<br>
Q13: Treatment of (+)-(R)-3-methyl-1-pentene with HBr and peroxides